15, April 2019 – Daily Current Affairs
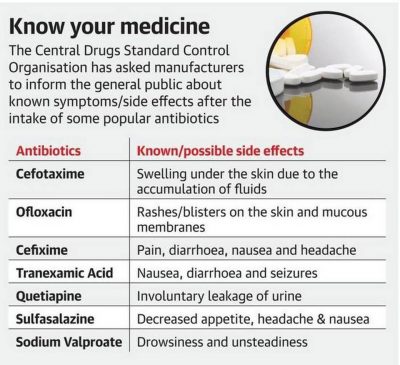
Pharmacovigilance Programme of India For: Preliminary Topic: Pharmacovigilance initiatives in India News Flash The Central Drugs Standard Control Organisation (CDSCO) has asked commonly-used antibiotics manufacturers to ensure its details be made available to the general public. This decision was taken considering directives from the National Co-ordination Centre of the Pharmacovigilance Programme of India (PvPI). Pharmacovigilance Programme of India The Pharmacovigilance Program of India (PvPI) was launched with a broad objective to safe guard the…
Continue reading
 Continue reading...
Continue reading...